Medication Guide
Introduction to FLEXISEQ
FLEXISEQ is a novel medical device for the relief of joint pain that has been examined in several phase III trials, across more than 1000 osteoarthritis patients, for up to 12 months.

- FLEXISEQ was very effective in reducing pain. In a particular study including a comparator arm using oral celecoxib, FLEXISEQ showed superiority to the oral placebo and demonstrated efficacy and response rates comparable to those reported for the celecoxib treatment arm; and
- FLEXISEQ demonstrated an incidence of minor side effects in approximately 1 in 10 patients which were mainly mild dermal irritations.
FLEXISEQ®: Effective Pain Relief
The results of the most recent phase III trials showed that topically applied FLEXISEQ was clinically effective in reducing pain and restoring joint function (mobility).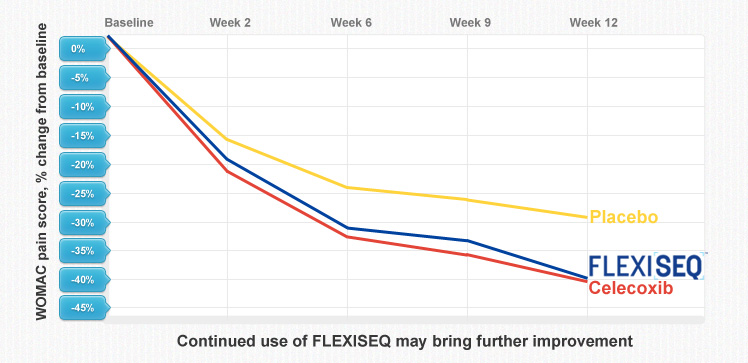
- Adverse drug interactions
To date, no adverse drug interactions have been reported or demonstrated with FLEXISEQ - A medical device with no pharmacological action.
Experiments with different formulations of FLEXISEQ have shown that the device does not interfere with key enzymatic complexes. - A non-systemic product
Most drug products, no matter what their mode of administration (e.g. oral or topical), will ultimately be absorbed and transported by the bloodstream systemically around the body prior to exerting their effect. Not only does this dilute the effect of the product away from the site where it is needed, but can also deliver the product to parts of the body where it is undesired and may cause harm.
The Sequessome Technology® at the heart of FLEXISEQ is different.
Experiments using Sequessome vesicles labelled with a marker molecule (cyclosporine) have shown that after local topical application of the product, the marker molecule was later accumulating in the lymph system and not becoming widely systemically distributed as might be expected with naked cyclosporine. In other trials where Sequessome vesicles have been loaded with other chemicals (e.g. Ketoprofen) serum samples have shown that the chemicals did not enter the blood stream except in tiny quantities that could only just be measured.
